【縛雞之見】
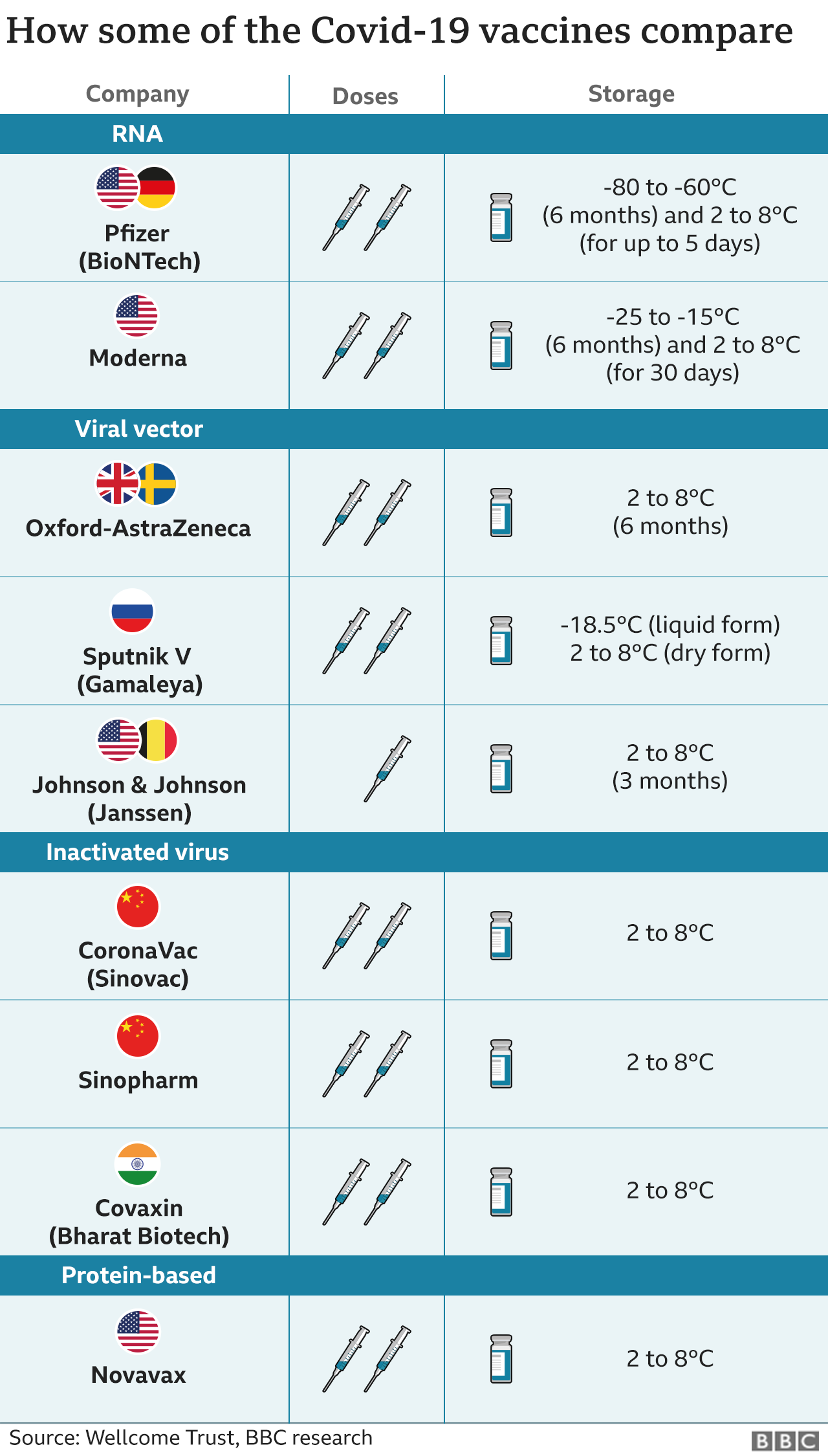
WHO approved the Chinese inactivated virus vaccine Sinopharm, meaning
Taiwan, which holds few AZ vaccines that are far less than needed to produce herd
immunity and are expired by mid-June, has faced disturbing pressure to purchase
Sinopharm.
The inactivated virus vaccine, or attenuated vaccine, such as like
Sinophram, utilizes real viruses, only they are dead or reducing the virulence
of a pathogen. For the SARS-Cov-2 is a novel virus, it is difficult to
comprehend the whole mechanism of what will happens once the inactivated virus
enters into human bodies.
We worry that Chinese authorities reluctant to share the pandemic data, the
development of the vaccine is not transparent. How could we trust the
attenuated vaccine developed in such a country?
The inactivated virus is an old-fashioned technology that ancient India has
introduced for the fighting of Smallpox. The modern smallpox vaccine, using the
live virus, was invented in the 18th century.
It is not to say that old-fashioned technologies are inferior. The risk is that
the virus does not inactivate completely—the vaccination leads itself to the
infection.
世卫组织批准了中国的去活性病毒疫苗国药集团,这意味着台湾持有的AZ疫苗很少,远远低于产生群体免疫力所需的疫苗,而且在6月中旬就会过期,台湾面临着购买国药集团的令人不安的压力。
去活性病毒疫苗,或称减毒疫苗,比如像國耀疫苗,利用的是真正的病毒,只是它们已经死亡或降低了病原体的毒力。由于SARS-Cov-2是一种新型病毒,很难理解灭活病毒进入人体后後的完整机制。
我们担心中国当局不愿意分享大流行病的数据,疫苗的开发也不透明。我们怎么能相信在这样一个国家开发的减毒疫苗?
去活性病毒是一种古老的技术,是古印度为对抗天花而引进的。使用活病毒的现代天花疫苗是在18世纪发明的。
这并不是说老式的技术是劣质的。风险在于病毒没有完全灭活--接种疫苗本身导致了感染。(中文由DeepL翻譯,Taimocracy修正)
Sinopharm:
Chinese Covid vaccine gets WHO emergency approval BBC 20210508
沒有留言:
張貼留言
請網友務必留下一致且可辨識的稱謂
顧及閱讀舒適性,段與段間請空一行